- >Discover our teams
- >CarMe
- >Ion channels in vascular function
Ion channels in vascular function
Christian Legros, Professor

christian.legros@univ-angers.fr
People involved in the project
- Christian Legros, Ph.D. (Professor, https://orcid.org/0000-0002-3346-7059)
- César Mattei, Ph.D. (Associate-Professor, https://orcid.org/0000-0002-2214-2611)
- Hélène Tricoire, Ph.D. (Associate-Professor)
- Claire Legendre, Ph.D (Associate-Professor)
- Florian Beignon (Post-doc)
- Léa Réthoré (Ph.D. Student)
- Léa Tuifua (Ph.D. Student)
- Christina Sahyoun (Ph.D. Student)
- Eliska Daďová (Ph.D. Student)
- Jennifer Bourreau (Assistant-Ingenior, INSERM)
- Cyril Le Corre (Technical assistant)
State of the art and objectives
Our group is interested in elucidating the role of voltage-gated sodium channels in the vascular function. We are currently working on different aspects of the cellular and molecular pharmacology of voltage-gated sodium channels (alkaloids, animal toxins…), the cell signalling they induce – particularly regarding the intracellular Ca2+ homeostasis – and their role in different cell types (endocrine cells, vascular endothelial cells…). Some of our projects also involve the pharmacological and physiological implication of GABA receptors and nicotinic acetylcholine receptors as key players of the vascular function and the regulation of the arterial blood pressure.
Project supported by
- DGA/MinDef (bourse de thèse 2018-2021 – Florian Beignon ; bourse de thèse Léa Tuifua 2022-2025)
- MESR (bourse de thèse 2019-2022 – Léa Rethoré)
- ANR (projet TempoMito 2022-2025 – César Mattei)
- SFR-ICAT (projet PROTEOSHEAR – Claire Legendre)
- Prix encouragement de la Fondation Grand-Ouest (projet MECANONAV 2022 – Claire Legendre)
- FRM (prolongation de thèse – Léa Rethoré)
Collaborations
- Nathalie Guérineau, Arnaud Monteil (IGF Montpellier)
- Antoine Taly (Institute of Physico-Chemical Biology, CNRS UPR9080, Paris)
- Marc Verleye (Biocodex, Gentilly)
- Benjamin Marie (Muséum National d’Histoire Naurelle)
- Mireille Chinain (Institut Louis Mallardé, Polynésie française)
- Jordi Molgó (CNRS-CEA Saclay)
- Nathalie Arnich (ANSES Maisons-Alfort)
- Andrea Bourdelais (Level 1 Fasteners, Wilmington, USA)
- Ziad Fajloun (Université Libanaise, Liban)
- Petr Humpolíček (Université Thomas Bata, République Tchèque)
- Marie Philips (University of Melbourne, Australia)
Découverte de nouveaux alcaloïdes de plantes possédant des potentiels thérapeutiques.
Review articles:
- Sahyoun C, Rima M, Mattei C, Sabatier J-M, Fajloun Z, Legros C (2022). Separation and Analytical Techniques Used in Snake Venomics: A Review Article. Processes 2022, 10(7), 1380; https://doi.org/10.3390/pr10071380.
- Beignon F, Gueguen N, Tricoire-Leignel H, Mattei C, Lenaers G. (2022). The multiple facets of mitochondrial regulations controlling cellular thermogenesis. Cell Mol Life Sci. 79(10):525; https://doi.org/10.1007/s00018-022-04523-8.
- Mackieh R, Abou-Nader R, Wehbe R, Mattei C, Legros C, Fajloun Z, Sabatier JM. (2021). Voltage-Gated Sodium Channels: A Prominent Target of Marine Toxins. Mar Drugs. 19(10): 562 ; https://doi.org/10.3390/md19100562.
- Frangieh J, Rima M, Fajloun Z, Henrion D, Sabatier JM, Legros C, Mattei C. (2021). Snake Venom Components: Tools and Cures to Target Cardiovascular Diseases. Molecules. 26(8): 2223 ; https://doi.org/10.3390/molecules26082223.
- Colas S, Marie B, Lance E, Quiblier C, Tricoire-Leignel H, Mattei C. (2021). Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ Res. 193: 110590.
- Delcourt N, Lagrange E, Abadie E, Fessard V, Frémy JM, Vernoux JP, Peyrat MB, Maignien T, Arnich N, Molgó J, Mattei C (2019). Pinnatoxins' Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health. Mar Drugs. 17(7): 425.
- Mattei C (2018). Tetrodotoxin, a Candidate Drug for Nav1.1-Induced Mechanical Pain? Mar Drugs. 16(2):72.
- Delcourt N, Claudepierre T, Maignien T, Arnich N, Mattei C (2017). Cellular and Molecular Aspects of the β-N-Methylamino-l-alanine (BMAA) Mode of Action within the Neurodegenerative Pathway: Facts and Controversy. Toxins (Basel). 10(1): 6.
Research articles:
- Park J, Proux C, Ehanno W, Réthoré L, Vessières E, Bourreau J, Favre J, Kauffenstein G, Mattei C, Tricoire-Leignel H, Henrion D, Legendre C, Legros C (2023). Tetrodotoxin Decreases the Contractility of Mesenteric Arteries, Revealing the Contribution of Voltage-Gated Na+ Channels in Vascular Tone Regulation. Mar. Drugs 2023, 21(3), 196; https://doi.org/10.3390/md21030196.
- Sahyoun C, Krezel W, Mattei C, Sabatier JM, Legros C, Fajloun Z, Rima M. Neuro- and Cardiovascular Activities of Montivipera bornmuelleri Snake Venom. Biology (Basel). 2022 Jun 9;11(6):888; https://doi.org/10.3390/biology11060888.
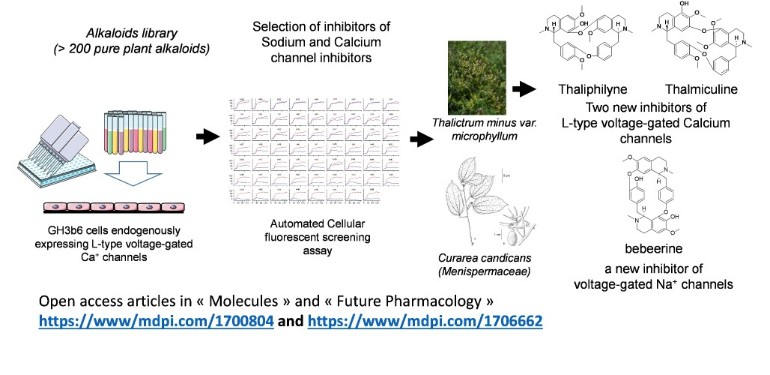
- Frangieh J, Legendre C, Bréard D, Richomme P, Henrion D, Fajloun Z, Mattei C, Le Ray AM, Legros C. Oxostephanine, Thalmiculine, and Thaliphyline—Three Isoquinoleine Alkaloids That Inhibit L-Type Voltage-Gated Ca2+ Channels. Future Pharmacol. 2022 2(3), 238-255; https://doi.org/10.3390/futurepharmacol2030016.
- Coquerel Q, Legendre C, Frangieh J, Waard S, Montnach J, Cmarko L, Khoury J, Hassane CS, Bréard D, Siegler B, Fajloun Z, De Pomyers H, Mabrouk K, Weiss N, Henrion D, Richomme P, Mattei C, Waard M, Le Ray AM, Legros C. Screening an In-House Isoquinoline Alkaloids Library for New Blockers of Voltage-Gated Na+ Channels Using Voltage Sensor Fluorescent Probes: Hits and Biases. Molecules. 28;27(13):4133; https://doi.org/10.3390/molecules27134133.
- Réthoré L, Park J, Montnach J, Nicolas S, Khoury J, Le Seac'h E, Mabrouk K, De Pomyers H, Tricoire-Leignel H, Mattei C, Henrion D, Fajloun Z, De Waard M, Legendre C, Legros C. (2022). Pharmacological Dissection of the Crosstalk between NaV and CaV Channels in GH3b6 Cells. Int J Mol Sci. 23(2): 827 ; https://doi.org/10.3390/ijms23020827.
- Soualah Z, Taly A, Crespin L, Saulais O, Henrion D, Legendre C, Tricoire-Leignel H, Legros C, Mattei C. (2021). GABAA Receptor Subunit Composition Drives Its Sensitivity to the Insecticide Fipronil. Front Neurosci. 15: 768466.
- Park J, Taly A, Bourreau J, De Nardi F, Legendre C, Henrion D, Guérineau NC, Legros C, Mattei C, Tricoire-Leignel H. (2021). Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure. Int J Mol Sci. 22(10):5106 ; https://doi.org/10.3390/ijms22105106.
- Arnich N, Abadie E, Amzil Z, Dechraoui Bottein MY, Comte K, Chaix E, Delcourt N, Hort V, Mattei C, Molgó J, Le Garrec R. (2021). Guidance Level for Brevetoxins in French Shellfish. Mar Drugs. 19(9): 520.
- Arnich N, Abadie E, Delcourt N, Fessard V, Fremy JM, Hort V, Lagrange E, Maignien T, Molgó J, Peyrat MB, Vernoux JP, Mattei C. (2020). Health risk assessment related to pinnatoxins in French shellfish. Toxicon. 180: 1-10.
- Mattei C, Taly A, Saulais O, Henrion H, Guérineau NC, Verleye M, Legros C (2019). Involvement of the GABAA receptor alpha-subunit in the mode of action of etifoxine. Pharmacological Research. 145:104250.
- Frangieh J, Salma Y, Haddad K, Mattei C, Legros C, Fajloun Z, El Obeid D (2019). First Characterization of The Venom from Apis mellifera syriaca, A Honeybee from The Middle East Region. Toxins (Basel). 11(4). 191.
- Battault S, Meziat C, Nascimento A, Braud L, Gayrard S, Legros C, De Nardi F, Cazorla O, Drai J, Thireau J, Meyer G, and Reboul C (2018). Vascular endothelial function masks increased sympathetic vasopressor activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. H-00217-2017R1.
- De Nardi F, Lefort C, Bréard D, Richomme P, Legros C*, Guérineau NC* (2017). Monitoring the secretory behavior of the rat adrenal medulla by high-performance liquid chromatography-based catecholamine assay from slice supernatants. Front Endocrinol (Lausanne). 25;8:248.
- Khoury J, Dabbousya R, Riyad S, Antound S, Hleihele W, Legros C*, Fajloun Z* (2017). Evidence for in vitro antiophidian properties of aqueous buds extract of Eucalyptus against Montivipera bornmuelleri venom. J of Venom Res. 18;8:25-30.
Editorials:
- Mattei C, Tricoire-Leignel H, Legros C, Lewis RJ, Molgó J. (2022). Editorial: Pharmacological Aspects of Ligand-Gated Ion Channels as Targets of Natural and Synthetic Agents. Front Neurosci. 16:895299. doi: 10.3389/fnins.2022.895299.



