- >Discover our teams
- >CarMe
- >Arterio-venous relations in physiopathological conditions
Arterio-venous relations in physiopathological conditions
Laurent Loufrani, Dr2 Inserm

laurent.loufrani@inserm.fr
Keywords : cardiovascular system, arteries, veins, hypertension, aneurysm, atherosclerosis.
Preclinical Team:
Laurent Loufrani, PhD, CNRS researcher
Linda Grimaud, assistant-engineer
Agnès Barbelivien, technician
Clément Tétaud, technician
Pauline Robert, PhD Student
Alexis Richard, PhD Student
Clinical Team:
Christophe Baufreton, M.D, Ph.D, CHU Angers
Olivier Fouquet, M.D., Ph.D, CHU Angers
Maroua Eid, Master Student
Since 2015 we focused our research on the vascular remodeling by the pressure and the blood flow (hypertension and atherosclerosis). According to the World Health Organization, hypertension is estimated to cause yearly 7.5 million deaths, 12.8% of the total deaths worldwide. Blood pressure levels are positively and correlated to the risk for stroke, coronary heart disease, atherosclerosis and aneurysm. In addition, complications of raised blood pressure include heart failure, peripheral vascular disease, renal impairment, retinal hemorrhage and visual impairment.
It has been recently proposed that immune system and ROS of mitochondrial origin play an important role in the development of systemic hypertension. Nevertheless, mitochondrial involvement in the cardiovascular system and its pathophysiological role in systemic hypertension and the outcomes remain unclear.
Furthermore, veins are often associated with arteries through perivascular fat. They express a complex system of adhesive proteins, chemokines, and specialized cell-to-cell junctions that can regulate the passage of immune cells from veins to the arteries. Furthermore, artery needs also immune cells recruitment in many phenomena, sometimes physiological as remodeling or sometimes pathological as atherosclerosis or aneurysm.
The project aims at deciphering the mechanism(s) of this involvement of the venous system in the adaptation of the arterial system to changes in hemodynamic environment. Our goal is to discover how these two systems communicate and collaborate in order to activate the immune system involved in remodeling and how they involve the mitochondria.
First axis: Hypertension and mitochondrial dynamics

A relevant role of mitochondrial dynamics in the physiology of vascular cells has been evoked, showing that the outer membrane fusion protein MFN2 plays a crucial role in vascular cells function and viability. OPA1 is the protein responsible for the inner membrane fusion and its loss leads to mitochondrial fragmentation and cristae disorganization, subsequently resulting in reduced ATP synthesis and increased ROS production, altered calcium homeostasis, and inhibition of cell growth and susceptibility to apoptosis. In this respect, mitochondria dynamic could contribute to vascular hypertension.
Second axis: Aortic aneurysm and mitochondrial dynamics
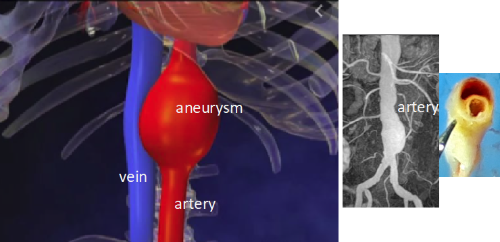
Aorta, the largest artery located between the heart and peripheral arteries, is always submitted to strong hemodynamic modifications. Depending of the distance from the heart, this impacts the aortic wall structure and may cause atheroma, aneurysm or aortic dissection in different aorta location. The consequences of aneurysm and dissection are now well established but the mechanisms causing these pathologies remain unknown.
Third axis: Essential interaction of veins and arteries in atherosclerosis and aortic aneurysm
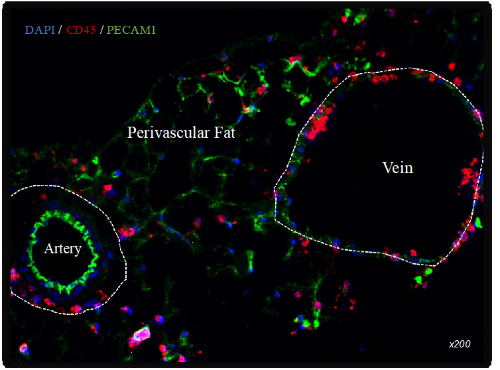
This axis focuses on tle of the vein in arterial remodeling and the involvement of the vein in the development of inflammation associated with arterial pathologies. The signaling pathways involved will be analyzed by immunolabelling using intravital biphotonic microscopy, biochemistry and molecular biology. In addition, veins and arteries are surrounded by perivascular fat whose role in the communication between arteries and veins and in arterial remodeling will also be analyzed by the same approaches.
Output: this project should lead to a better understanding of the mechanisms involved in the development of arterial and cardiovascular diseases with the hope of being able to consider the development of new treatments to prevent or reduce the occurrence of major cardiac or cerebrovascular accidents.
Collaborations
- A. Chevrolier (Mitolab): Analysis of mitochondrial dynamics.
- G. Loirand, V. Sauzeau (Inserm 1087, Nantes) Signaling of vein-artery interaction.
- O. Blanc-brude (PARCC, INSERM U970, Paris) Signaling of vein-artery interaction.
- B. Cariou, X, Prieur (Inserm 1087, Nantes), role of perivascular fat in vein-artery interaction.
- Muriel Laffargue (Inserm 1048, Toulouse), role of the venous endothelial cell in arterial adaptation.


